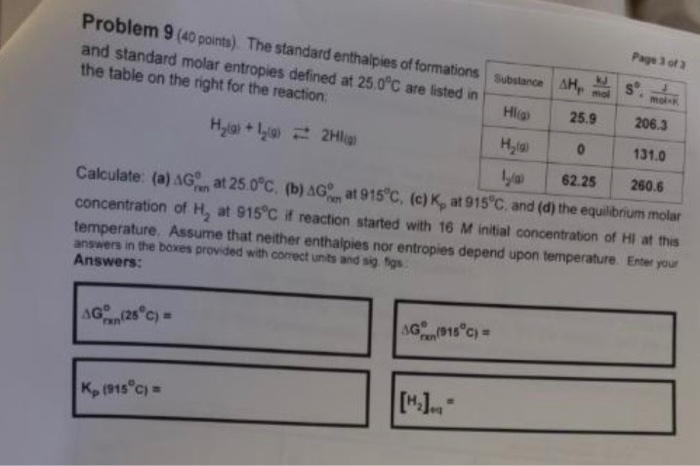
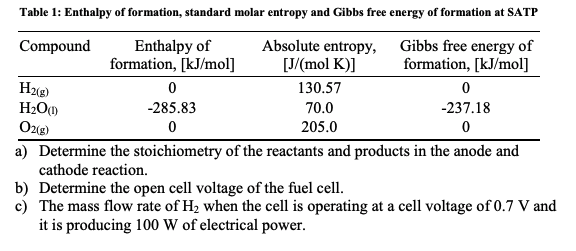
Standard Molar Entropy Table : Table I from Large-scale calculations of gas phase ... - These values have been tabulated, and selected substances are listed in table 18.1 standard molar entropies of selected substances at 298 k.
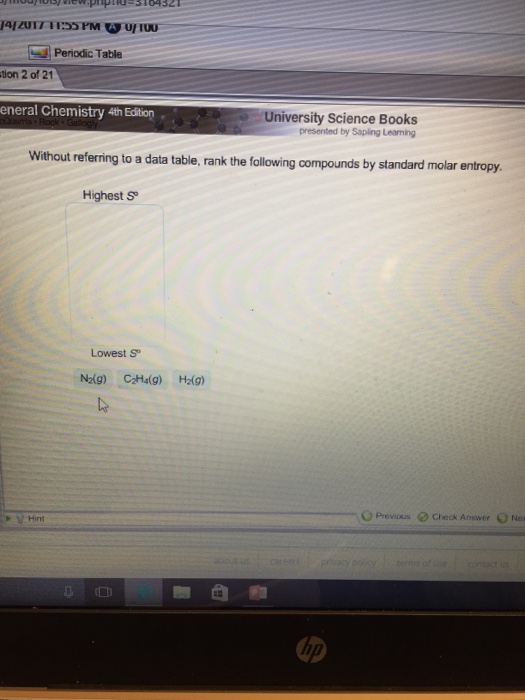
Standard Molar Entropy Table : Table I from Large-scale calculations of gas phase ... - These values have been tabulated, and selected substances are listed in table 18.1 standard molar entropies of selected substances at 298 k.. In chemistry, the standard molar entropy is the entropy content of one mole of pure substance under a standard state (not standard the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Standard molar entropy—denoted with s0—represents the entropy of one mol of pure substance at 25°c. Without referring to a data table, rank the following compounds by standard molar entropy (highest to lowest). The standard molar entropy is given the symbol s°, has units of joules per mole kelvin. Thank you for your help.
Shields explains the third law of thermodynamics and how to use standard molar entropy values in calculating entropy changes for chemical reactions. Nitrogen entropy table change in entropy table standard enthalpy table standard density table standard water table standard electric potential table standard probability table standard reduction potential table standard work table standard correlation table standard temperature. Entropy is also dependent upon volume, but since the amount, n, temperature. Calculate the standard molar entropy at (a) 100°c and (b) 500°c. This table gives the standard state chemical thermodynamic properties of about 2400 individual substances in the crystalline, liquid, and gaseous states.
Standard molar entropy is defined as the entropy or degree of randomness of one mole of a sample under standard state conditions.
When heat energy is input into a system, it introduces some level of entropy. Shields explains the third law of thermodynamics and how to use standard molar entropy values in calculating entropy changes for chemical reactions. Thank you for your help. Standard molar entropy in chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard the standard molar entropy is usually given the symbol so, and the units j mol−1 k−1 (joules per mole kelvin). The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). In chemistry, the standard molar entropy is the entropy content of one mole of pure substance under a standard state (not standard the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Furthermore, at 0 k, the standard molar entropy is nil because at this state the substance is represented by a perfect crystalline structure of zero randomness state. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a compound from its see also standard enthalpy of formation, gibbs free energy of formation, entropy and molar heat capacity of organic substances and thermodyamics key. Unlike standard enthalpies of formation, the value. Standard molar entropy of a substance is the actual entropy content of 1 mole of a substance under standard conditions. I can do it with a data table but not sure how to proceed without. Fra wikipedia, det frie encyklopædi. Entropy of any substance at 0k = nearly 0 (negligible).
Such conditions need to be specificed, since entropy is propotional to substance amount, and dependent on temperature, pressure. It goes by weight, so in order of increasing standard molar entropy, it would go from the lowest weight to the heaviest weight. Thank you for your help. The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j mol−1 k−1). Standard molar entropy—denoted with s0—represents the entropy of one mol of pure substance at 25°c.
Such conditions need to be specificed, since entropy is propotional to substance amount, and dependent on temperature, pressure.
When heat energy is input into a system, it introduces some level of entropy. The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Fra wikipedia, det frie encyklopædi. Thank you for your help. The standard molar entropy, so, is the entropy of 1 mole of a substance in its standard state, at 1 atm of pressure. The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j mol−1 k−1). Table 2.13 standard molar entropies of some main group elements and the conventional standard molar entropies of their aqueous cations at 25 c (in j k 1 mol A table of standard molar entropies at 0k would be pretty useless because it would be 0 for every substance (duh!) Standard molar entropy is defined as the entropy or degree of randomness of one mole of a sample under standard state conditions. This table shows molar entropies for the standard conditions of 298.15 k (25°c) and 101.3 kpa. For a substance at 0k to be brought to. 1 the standard molar entropies of selected substances at 298.15 k (25°c) compound. Furthermore, at 0 k, the standard molar entropy is nil because at this state the substance is represented by a perfect crystalline structure of zero randomness state.
The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Third law of thermodynamics room temperature dislocations. Nitrogen entropy table change in entropy table standard enthalpy table standard density table standard water table standard electric potential table standard probability table standard reduction potential table standard work table standard correlation table standard temperature. Thank you for your help. Unlike standard enthalpies of formation, the value of s° is absolute;
The standard molar entropy, so, is the entropy of 1 mole of a substance in its standard state, at 1 atm of pressure.
In chemistry, the standard molar entropy is the entropy content of one mole of pure substance under a standard state (not standard the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). A table like this can be used in much the same way as a table of standard enthalpies of formation in order to find the entropy change δ sm ° for a reaction occurring at standard pressure and at 298 k. It goes by weight, so in order of increasing standard molar entropy, it would go from the lowest weight to the heaviest weight. The entropy of a substance has an absolute value of 0 entropy at 0 k. Entropy is also dependent upon volume, but since the amount, n, temperature. 3.10 a gaseous sample consisting of 1.00 mol molecules is described by the equation of state pvm. Nitrogen entropy table change in entropy table standard enthalpy table standard density table standard water table standard electric potential table standard probability table standard reduction potential table standard work table standard correlation table standard temperature. ∗the standard entropy of the h+(aq) ion is dened to be 0. Entropy of any substance at 0k = nearly 0 (negligible). The standard molar entropy is given the symbol s°, has units of joules per mole kelvin. When heat energy is input into a system, it introduces some level of entropy. That is, an element in its standard state has a definite therefore, a table of values for c p k t is required to find the total molar entropy. Shields explains the third law of thermodynamics and how to use standard molar entropy values in calculating entropy changes for chemical reactions.
Komentar
Posting Komentar